WELCOME TO
PHOTONMED
We accelerate the uptake of photonics technologies in medical device applications.


overview
The PhotonMed project advances photonics technologies for innovative medical devices. The application relevance of the PhotonMed research and its quality are validated in 16 pilot-cases led by the end-user companies. Based on the pilot-cases, industrial supply chains are defined for the concepts of the end-user companies reducing substantially the time-to-market. Technologies developed in PhotonMed will be appended also to the pilot line offering making them available to all companies outside PhotonMed via open access policy.
The pilot line R&D model, exploited in PhotonMed, was successfully established in the previous EU funded MedPhab project. The model is the core of an operational MedPhab pilot line with RTO and industrial members across Europe. The research results from PhotonMed will be immediately exploited by the end-user companies in their R&D towards the product launches. In the long-term, the effective exploitation of the advanced technologies by RTOs and industrial suppliers is ensured by appending them to the pilot line offering.
our objectives
The development of advanced photonics technologies is driven by the need for medical applications in diagnostics and personalized monitoring. Research work focuses on new components, integration technologies and new processes units for pilot line fabrication.
- The project aims to introduce 10 new photonics component technologies, 11 integration technologies, and 3 new assembly units for pilot line fabrication
PhotonMed develops technologies up to TRL5 demonstrators, ensuring industry relevance through end-user company validation. The process involves a requirement-to-validation chain with stakeholder collaboration, aiming for industry-controlled fabrication. Project aims to include novel technologies in 16 pilot cases across three medical device fields, utilization of eight technology value chain blocks, and three advanced production equipment types.
Significant delays in the uptake of photonics technologies are due to issues in knowhow, fragmented technology offerings, and regulatory topics. The accelerated process relies on a pilot line concept involving multiple RTO and industrial partners
- By considering quality management and regulation early in the development phase, gaps can be avoided later, leading to faster adoption of research results
- Technologies developed in PhotonMed are included in the sustainable pilot line offering, ensuring long-term applicability beyond the scope of PhotonMed
- Technology production chains established, risk management for the end-users
- End-user companies create updated plans for the accelerated product development
- The PhotonMed project aims to create significant business opportunities for stakeholders by establishing technology value chains and building TRL5 demonstrators
- Industrial suppliers benefit from more mature cases from RTO-driven projects, simplifying the transfer from RTO to industry when regulatory aspects are addressed in the research phase
- Innovative medical device companies benefit from insights into available technologies, established development chains, and guidance on regulatory topics
- New business opportunities for project partners and external companies
application domains

In vitro diagnostics
pilot system manufacturing and validation
7 pilot cases
Pilot case application examples include In Vitro Diagnostics (IVD) such as DNA analytics and organ-on-chip technologies for drug development.

In-vivo diagnostics
pilot system manufacturing and validation
7 pilot cases
In-vivo applications encompass surgery assistance and the use of catheters for monitoring cardiovascular diseases.

Personalized monitoring
pilot system manufacturing and validation
2 pilot cases
Personalized applications encompass spectral monitoring of physiological conditions in home settings.
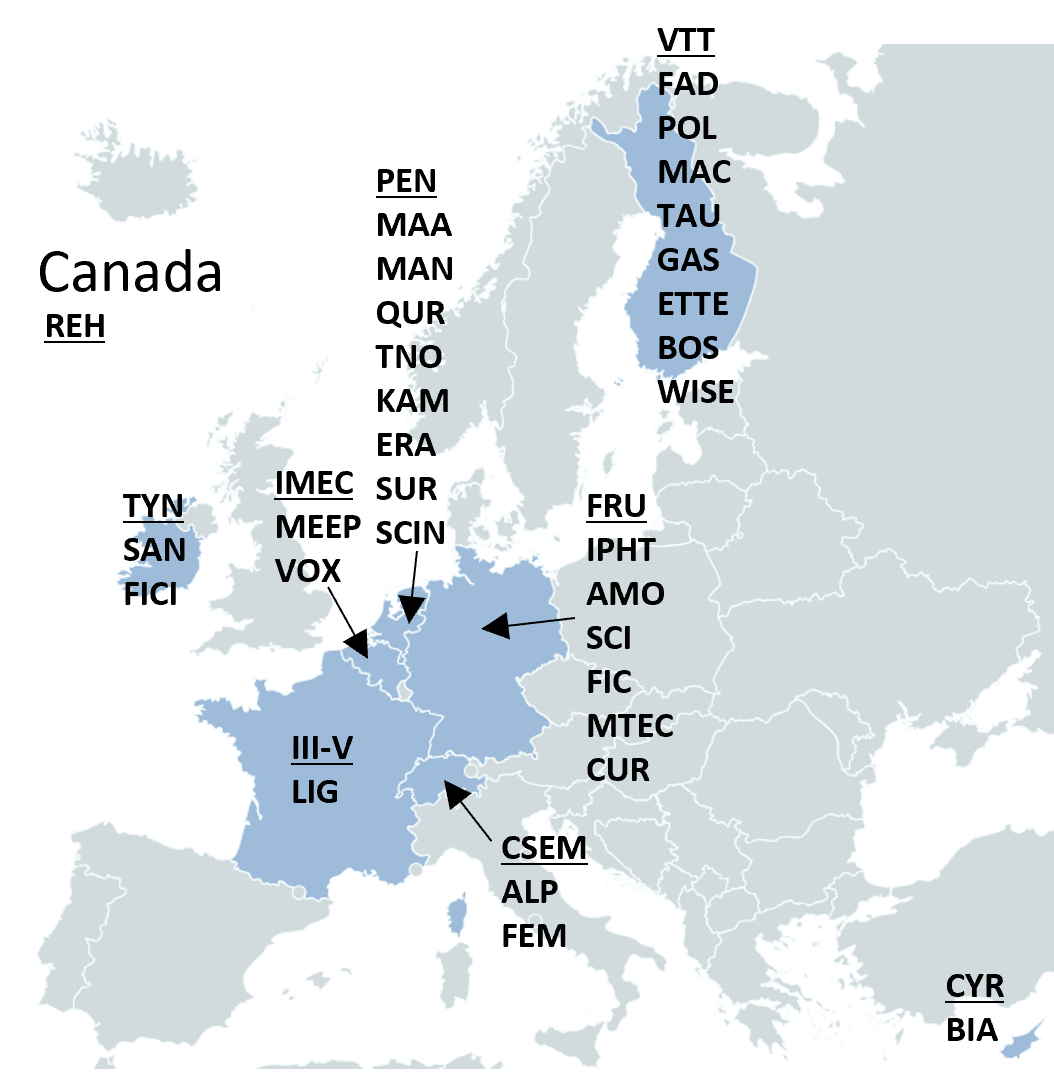
consortium
39 partners
Led by VTT Technical Research Center of Finland Ltd. Combination of large enterprises, SMEs, research organizations and universities to cover full development chain from concepting to industrial fabrication.
9 countries
Belgium, Cyprus, Finland, France, Germany, Ireland, Netherlands, Switzerland, Canada.